Troxerutin
Introduction: Excerpted from the Chinese Pharmacopoeia 2015 edition of quercetin


Peak 1: Tetrahydroxyethyl rutin Peak 2: Monohydroxyethyl rutin Peak 3: Rutin Peak 4: Troxerutin Peak 5: Dihydroxyethyl rutin Peak
Chromatographic column:Veunsil®MP C18,5 μm,100 Å,4.6×250 mm, P/N:VA952505-0;
Mobile phase:Phosphate buffer solution with pH 4.4 (0.1mol/L sodium dihydrogen phosphate solution, adjusted to pH 4.4 with phosphoric acid) - acetonitrile (80:20);
Current Speed:1.0 mL/min; Injection volume: 10 μ L; Wavelength: 254 nm
Mecobalamin injection
Introduction: According to the 2015 edition of the Chinese Pharmacopoeia, the test results for methylcobalamin injection fully comply with the standard requirements.

High performance liquid chromatogram of the test substance related to methylcobalamin injection (the following figure is a partial enlargement of the above figure)
Chromatographic column:Durashell C18-AM,5 μm,100 Å,4.6×250 mm, P/N:DC952505-AM;
Mobile phase:0.03 mol/L potassium dihydrogen phosphate (adjusted to pH 4.5 with 0.2 mol/L sodium hydroxide): acetonitrile=84:16 (v/v);
Current Speed:1.2 mL/min; Injection volume: 20 μ L; Wavelength: 342 nm; Column temperature: 35 ℃
Cefmezole Sodium
Introduction: According to the 2015 edition of the Chinese Pharmacopoeia, the test results showed that the separation degree between 5-mercapto-1-methyltetrazolium and its precursor impurity (unknown impurity 1) was 5.18, the separation degree between 5-mercapto-1-methyltetrazolium and cefmetazole was 12.49, and the separation degree between cefmetazole and its subsequent impurity (unknown impurity 2) was 4.14. No impurity peaks were detected between 5-mercapto-1-methyltetrazolium and cefmetazole, all of which met the standard requirements.

Sample with hydrogen peroxide solution test diagram
The peak order is: hydrogen peroxide; Unknown impurity 1; 5-mercapto-1-methyltetrazolium; Cefmezole; Unknown impurity 2
Chromatographic column:Venusil®XBP C18 (L),5 μm,150 Å,4.6×250 mm, P/N:VX952505-L;
Mobile phase:Ammonium dihydrogen phosphate solution tetrahydrofuran methanol (730:12.5:300, v/v/v) (pH adjusted to 4.5 with phosphoric acid), isocratic elution;
Current Speed:1.0 mL/min; Injection volume: 20 mL; Detection wavelength: 254 nm; Column temperature: 35 ℃
Hydroxyphenethyl ester
Introduction: The test was conducted in accordance with the 2015 version of the Chinese Pharmacopoeia draft for soliciting opinions on cefmetazole sodium, and the results fully met the standard requirements.

System suitability liquid chromatography chart
The peak order is: hydroxyphenyl methyl ester; Hydroxyphenethyl ester
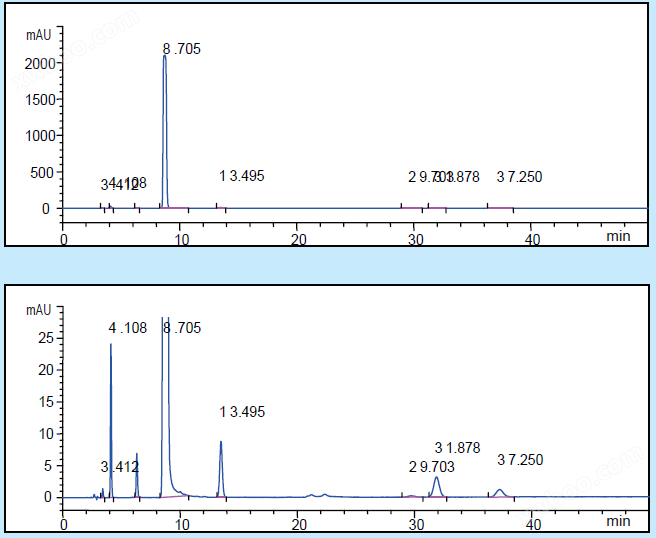
High performance liquid chromatography chart of the test solution for substances related to ethylparaben
(The following image is a partial enlargement of the previous image)
Chromatographic column:Venusil®MP C18,5 μm,100 Å,4.6×250 mmP/N: VA952505-0;
Current Speed:1.0 mL/min; Injection volume: 20 μ L; Column temperature: 30 ℃; Detection wavelength: 254 nm;
Chlorpromazine hydrochloride
Introduction: The 15th edition of the pharmacopoeia has specific regulations on the separation degree of bupropion hydrochloride in the 10th edition of the pharmacopoeia, and this test is conducted entirely according to the revised method; The results fully comply with the separation requirements in the revised standards.

High performance liquid chromatography (HPLC) chart of the applicability solution of bupropion hydrochloride system
The peak sequence is: phenylpropanol piperazine peak; Buquinine
Chromatographic column:Venusil®XBP C18 (L),5 μm,150 Å,4.6×250 mmP/N: VX952505-L;
Mobile phase:0.1mol/L ammonium acetate (ammonia test solution adjusted to pH 7.0): methanol=25:75 (v/v);
Current Speed:1.0 mL/min; Injection volume: 20 μ L; Column temperature: 30 ℃; Wavelength: 252 nm
Famotidine
Introduction: The opinion draft of the 15th edition of the Pharmacopoeia of Famotidine is divided into: 1. Famotidine Calcium Magnesium Chewable Tablets; 2 famotidine dispersible tablets; 3. Injection of famotidine. The methods for dealing with substances in liquid phase are the same. This test was conducted in accordance with the relevant substance methods in the pharmacopoeia, and the results fully meet the standard requirements.

High performance liquid chromatography image of the applicability solution of famotidine system (the following image is a partial enlargement of the above image)
The peak order is: impurity I; Famotidine; Impurity II
Chromatographic column:Durashell C18,5 μm,100 Å,4.6×250 mm, P/N:DC952505-0;
Mobile phase:A-Sodium acetate buffer: Acetonitrile=93:7 (v/v), B-Acetonitrile, gradient elution (according to pharmacopoeia);
Current Speed:1.5 mL/min; Injection volume: 20 μ L; Column temperature: 35 ℃; Wavelength: 270 nm;
Dexamethasone acetate
Introduction: The test was conducted in accordance with the methods of the 10th edition of the Chinese Pharmacopoeia, and the results met the standard requirements.


Chromatographic column:Innoval AQ C18,5 μm,100 Å,4.6×250 mm, P/N:IA952505-0;
Mobile phase:Water: Acetonitrile=40:60 (v/v);
Current Speed:1.0 mL/min; Injection volume: 20 uL;
Column temperature:30℃; Wavelength: 240 nm;


